With thousands of COVID-19 patients flooding Long Island’s two dozen hospitals and the need for ventilators turning desperate nationwide, a physician, a respiratory therapist, and a 3D printing bioengineer in Manhasset put their heads together and had a potentially life-saving eureka moment.
On March 31, the trio of Northwell Health staffers figured out how to convert a bilevel positive airway pressure (BiPAP) device — a widely used piece of medical equipment used to maintain consistent breathing for people with sleep apnea, congestive heart failure, and other ailments — into a ventilator. Upon learning of the development, Gov. Andrew Cuomo, who’s been rushing to increase the number of ventilators in New York State from about 3,000 to the 40,000 that could be needed when the virus is expected to peak in mid-April, ordered 7,000 BiPAP machines to help meet that goal.
“We’re still in it,” the governor said at one of his daily coronavirus news briefings while touting the idea and others, such as converting anesthesia machines into ventilators and having two patients per ventilator. “We’re creative and we’re working and figuring it out. And I’m still hopeful that at the end of the day we’ll have what we need.”
Despite the exponentially rising coronavirus caseload forcing hospitals to erect medical tents to triage patients, use refrigerated trucks to store the dead, and convert conference rooms into treatment areas, several LI innovators have offered glimmers of hope in the race to develop COVID-19 treatments, vaccines, and rapid-result tests.

TEST OF TIME
Testing is key to the strategy of identifying people who’ve contracted the virus, isolating them, and determining who they may have spread it to — but until recently, testing options were limited and results slow.
As of press time in early April, the Island had five drive-through test sites at Jones Beach State Park, Stony Brook University, and ProHEALTH Care’s urgent care locations in Jericho, Riverhead, and Lake Success.
“We don’t think this is gonna be a matter of weeks,” Dr. Zeyad Baker, president and CEO of ProHEALTH Care, told the Press in mid-March. He anticipates the need for such test sites will run for “a few months.”
The first private lab in the nation that the U.S. Centers for Disease Control and Prevention (CDC) authorized to perform COVID-19 tests last month was Northwell Lab, after the federal agency belatedly approved that the Empire State do its own testing, clearing up a national testing bottleneck at the CDC’s labs in Georgia. Once Northwell got the green light, it hit the ground running literally — opening the state’s first drive-through test site in the early hot spot of New Rochelle.
“At Northwell we have a phrase that we use … ‘We’re made for this,’” Northwell Health President and CEO Michael Dowling told reporters during the grand opening of that site. “We will deal with this issue practically … I have no doubt that we will be in the front lines of innovation, creativity, and eventually succeed.”
RUSH HOUR
While the drive-through sites help increase the volume of tests performed and identify more patients, several local companies are working to get tests that offer quicker results into the hands of those performing them.
Melville-based Henry Schein is shipping COVID-19 tests that can give results within 15 minutes from blood taken with a pinprick. The Standard Q COVID-19 IgM/IgG Rapid Test is made by South Korea-based SD Biosensor. Henry Schein had several hundred thousand tests available by March 30 and planned to significantly increase availability in April.
“This pandemic is an unprecedented situation and making rapid diagnostic tools available to healthcare professionals is critical for detecting and mitigating the spread of the coronavirus,” Henry Schein CEO Stanley Bergman said. “There is urgent need for rapid testing to quickly identify large numbers of previously infected patients, including asymptomatic carriers. This is important to reduce and prevent virus transmission, assure timely treatment of patients, and help return our citizens to the workforce.”
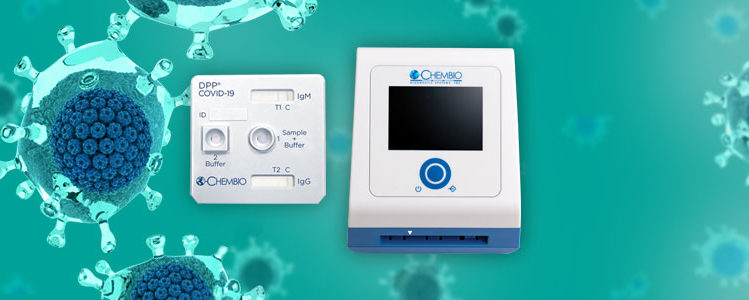
Hauppauge-based Chembio Diagnostics announced the launch of a 15-minute COVID-19 test in the United States on March 31. The company, which specializes in making tests for infectious diseases, said its rapid COVID-19 test detects antibodies related to the disease.
“We expect to begin shipping product in April 2020,” Chembio Diagnostics CEO Richard Eberly said.
Canon Medical Systems Corporation, a division of Melville-based Canon U.S.A., Inc., announced in March that it will start developing a rapid genetic testing system for the novel coronavirus in cooperation with Nagasaki University in Japan. It was not immediately clear how soon it’ll be available.
“The total time required is up to 40 minutes, including the time for pre-processing the sample,” a Canon spokeswoman said. “After preprocessing has been completed, the time required for detection in a positive sample is 10 minutes or less.”
THE PERFECT DRUG
Northwell Health has begun clinical trials of two medications designed to treat COVID-19, including one that seeks to attack the virus directly and another that targets the body’s own response.
The trials, in which people are already being enrolled, are being done through Northwell’s Feinstein Institutes for Medical Research at the system’s hospitals. The clinical trials are not open to the general public, but are enrolling patients in Northwell facilities.
Northwell, through its Feinstein Institutes of Clinical Research, is partnering with Foster City, Calif.-based Gilead Sciences on two clinical trials, looking at the safety and efficacy of remdesivir or RDV, an antiviral drug, to reduce the intensity and duration of COVID-19 in hospitalized patients.
The first trial can accommodate up to 400 severe cases of COVID-19 globally, with Dr. Marcia Epstein, Feinstein Institutes researcher and an infectious disease expert, as Northwell’s lead investigator.
Northwell is also taking part in a phase 3 study of RDV trial with Gilead that will look at up to 600 patients with moderate COVID-19 globally. Dr. Prashant Malhorta, assistant professor in the Institute of Health Innovations and Outcomes Research at Feinstein, is lead investigator for that study.
Northwell Health also initiated a third trial with up to 400 patients along with Eastview, N.Y.-based Regeneron Pharmaceuticals and Paris-based Sanofi, regarding the safety and efficacy of sarilumab.
Northwell Health described that study as focusing on “a human antibody that may prevent the activity” of interleukin-6 (IL-6) that may cause severe pneumonia in some COVID-19 patients.
MIRACLE CURE
While better tests and treatments are welcome news, the race to create a vaccine may be the most consequential of all. But one likely won’t be ready for at least a year to 18 months.
Among the researchers seeking a cure are experts at Farmingdale-based Codagenix, Inc., a clinical-stage biotechnology company working in collaboration with the Serum Institute of India to rapidly codevelop a vaccine against coronavirus using live COVID-19, known as live-attenuated vaccine.
“We are proud to be confronting this public health crisis head-on,” said Codagenix CEO J. Robert Coleman, Ph.D. “Live-attenuated vaccines like the ones developed by Codagenix are ideally suited to outbreak scenarios as they scale rapidly and generally require only modest amounts of active ingredient for each immunization, as compared to inactivated and subunit vaccines.”
With the number of coronavirus patients diagnosed on LI doubling from 10,000 to nearly 20,000 in the first week of April — a month after the first local case was confirmed — all eyes will be on these miracle workers as the crisis continues.
COVID-19 SUPPORT
New York State Coronavirus Hotline: 1-888-364-3065
NY COVID-19 Emotional Support Hotline: 1-844-863-9314
Nassau County text updates: Text COVID19NC to 888777
Suffolk County text updates: Text COVIDSUFFOLK to 67283
-With additional reporting by Adam Brownstein































